3.B] State and Explain Heisenberg’s Uncertainty principle and infer on the classical and quantum mechanical measurements.
Answer:-
Statement:
The simultaneous determination of the exact position and momentum of a moving particle is impossible.
Explanation :
According to this principle if Δx is the error involved in the measurement of position and ΔPx is the error involved in the measurement of momentum during their simultaneous measurement, then the product of the corresponding uncertainties is given by
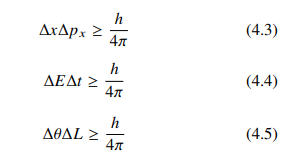
The product of the errors is of the order of Planck’s constant. If one quantity is measured with high accuracy then the simultaneous measurement of the other quantity becomes less accurate.
Infer on the classical and quantum mechanical measurements.
• Quantum mechanics is applied to microscopic bodies whereas classical mechanics is only applicable to macroscopic bodies.
• Quantum mechanics can be applied to macroscopic bodies but classical mechanics cannot be applied to microscopic systems.
• Classical mechanics can be considered as a special case of quantum mechanics.
• Classical mechanics is a fully developed field whereas quantum mechanics is still a developing field.
• In classical mechanics, most of the quantum effects such as energy quantization, uncertainty principal are not useful.

[…] Get Answer […]